


These light sources can be further subdivided into fluorescence sources and chemiluminescence.
We have created separate pages for the various light sources in our example spectra.
These sources for spectroscopic measurements are particularly suitable for the fields of physics, chemistry, materials science and, in some cases, biology.
Here, light is generated by absorption of excitation light (often UV) and subsequent emission in the visible spectrum—a phenomenon that is intensively studied in biochemistry, materials research and analytical chemistry.
Fluorescence is a fascinating phenomenon in which certain minerals emit light when irradiated with ultraviolet (UV) light. This property results from the ability of atoms or ions within the mineral structure to absorb light energy and re-emit it in the form of visible light. Fluorescent minerals not only provide spectacular visual effects, but also valuable information about the chemical composition and geological conditions under which they were formed. [More …]

In this experiment, we investigated the fascinating fluorescence of aesculin, a natural ingredient from the bark of the horse chestnut (Aesculus hippocastanum). Aesculin is known for its intense blue-green fluorescence, which is particularly visible under UV light excitation. [More …]

Here we present our latest sample spectra recorded with our DIY spectrometer. This time we have dedicated ourselves to the fascinating fluorescent colors of the Liqu-ment series from Colorberry. The intense colors neon green, neon yellow, neon tangerine, neon orange, neon pink and neon purple were analyzed under excitation with a 275 nm LED and reveal impressive spectra that make the luminosity of the fluorescent colors visible. [More …]

In this application note we present the recording of a spectrum of the fluorescence of »Lumogen Yellow S0790« with our DIY spectrometers. Lumogen is a fluorescent pigment based on azomethine, which is used in many applications as a downshifting material. [More …]

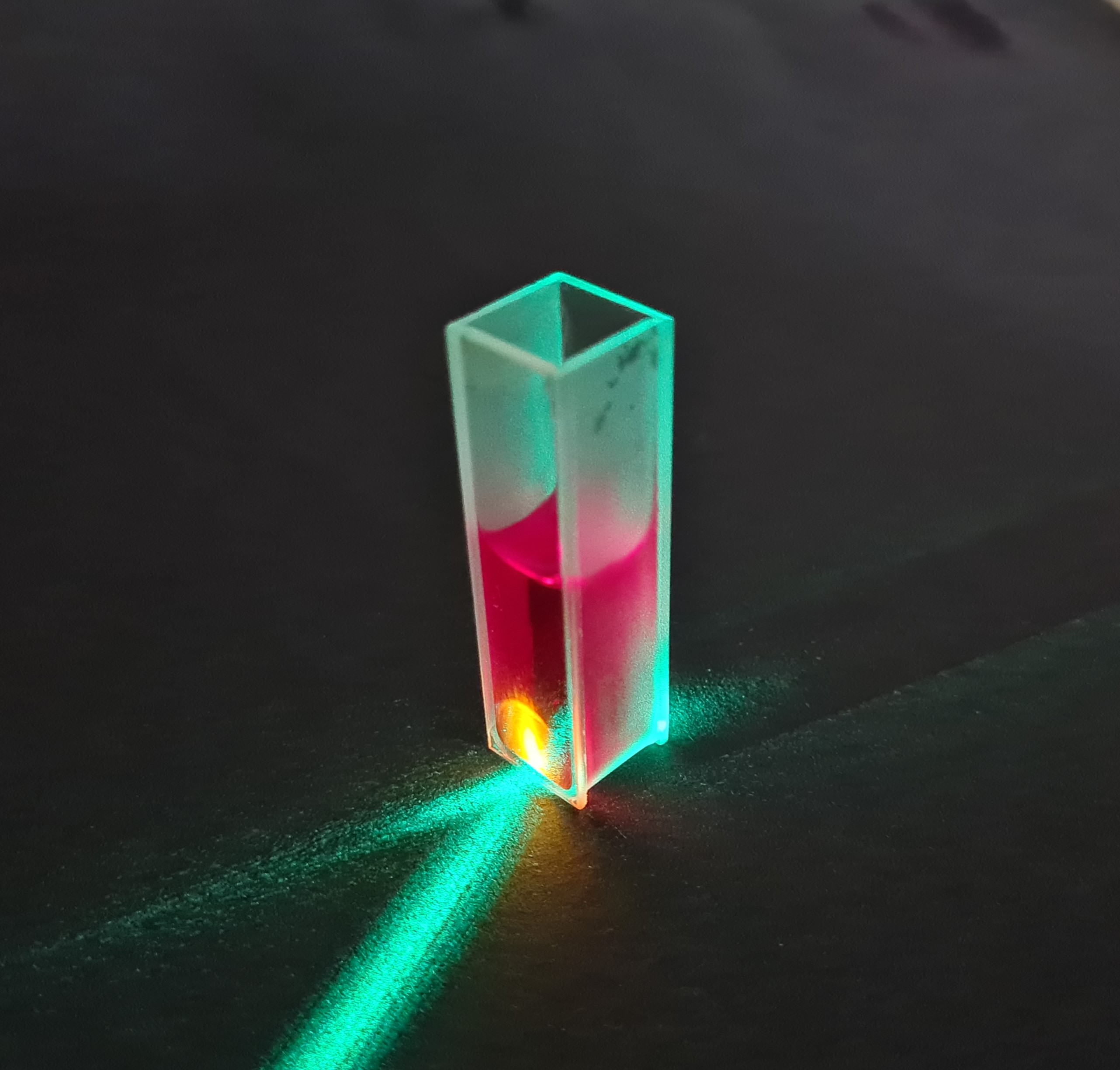
Rhodamine is a fluorescent dye that is widely used in many applications such as biochemistry, laser physics and microscopy. This application note shows how rhodamine in powder form can be prepared and measured for fluorescence measurement with our DIY spectrometers. The aim is to record a fluorescence spectrum and characterize the properties of rhodamine. [Mehr …]
Spectroscopy experiments with chemiluminescence sources are aimed at the fields of chemistry and physics. With these sources, light is released directly by chemical reactions without the need for an external light source.
This application description uses recorded spectra to show how different colors are produced in fireworks. Spectral experiments on fireworks are particularly popular with pupils and students because they are perceived as »cool«. This results in a high level of acceptance in practical courses, while at the same time the students learn a lot about emission lines of atomic origin or molecular bands. [More …]

Glow sticks are a fascinating example of chemiluminescence. This reaction is also responsible for the glow of many creatures, such as fireflies or deep-sea fish. Their biochemical variant is known as bioluminescence. Such creatures use the glow to communicate, find a partner or attract prey. [More …]

Here you can easily ask a question or inquiry about our products:
Last update: 2025-29-04







