


Fluorescence is a fascinating phenomenon in which certain minerals emit light when irradiated with ultraviolet (UV) light. This property results from the ability of atoms or ions within the mineral structure to absorb light energy and re-emit it in the form of visible light. Fluorescent minerals not only provide spectacular visual effects, but also valuable information about the chemical composition and geological conditions under which they were formed.
The fluorescence of minerals can appear in a variety of colors, from deep red and blue to vivid yellow and green. These colors are often the result of trace elements or defects in the mineral's crystal lattice that act as activators of fluorescence. For example, the presence of manganese, uranium, lead or other metal ions in low concentrations can promote fluorescence in specific colors.
Fluorescent minerals are not only of aesthetic interest; they also have practical applications in geology, mineralogy and other sciences. By studying fluorescent properties, researchers can gain valuable information about the formation and composition of rocks and deposits. These analyses also help to distinguish between different minerals and determine their origin.
In the following, we present a series of spectra of various minerals that fluoresce under UV light. The spectra were recorded with our DIY Czerny-Turner spectrometer CTS-150-C.

Unknown mineral

Fluorescence of the unknown mineral
The fluorescence was excited using UV LEDs with a wavelength of 275 nm, as these are now inexpensive and readily available. UV LEDs also have a longer service life and are more energy-efficient than other UV light sources. In addition, they generate less heat than mercury vapor lamps, for example, which results in a more stable operating environment and reduces the risk of overheating. LEDs are small and lightweight, making them easy to integrate into compact and portable fluorescence devices. This is particularly useful for field studies or mobile use.
In mineralogy, fluorescence excitation is traditionally realized by using mercury vapor lamps with a wavelength of 254 nm, which must be taken into account when comparing the spectra. The excitation efficiency of many minerals at 275 nm may be lower than at 254 nm. Since many classical studies and references in mineralogy are based on 254 nm, results obtained with 275 nm LEDs can only be compared to a limited extent.
Zircon is a widespread mineral that occurs in many different geological environments. It is a zirconium silicate mineral with the chemical formula ZrSiO₄ and is characterized by its high hardness and chemical resistance. Zircon can occur in a variety of colors, including colorless, brown, green, red and yellow. When irradiated with UV light, zircon often shows a yellow fluorescence, which is usually caused by the presence of trace elements such as uranium, thorium or rare earths (e. g. europium) in the zircon's crystal lattice. These elements partially replace the zirconium in the crystal structure and lead to electron transitions that emit light in the visible range. The intensity and color nuance of the fluorescence can vary greatly and depends on the concentration and type of trace elements contained.
Vishnevogorsk, located in the southern Ural Mountains in Russia, is a well-known site for various minerals, including zircon. The yellow fluorescence of zircon from Vishnevogorsk is particularly interesting, as it is more intense and consistent compared to zircon from other regions.

Zircon under daylight
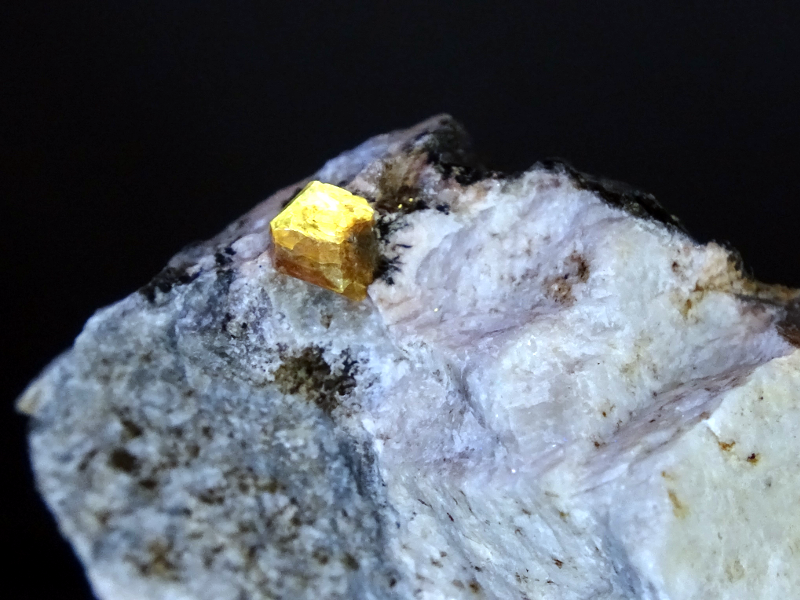
Zircon under UV light
Muscovite, a common mica mineral, belongs to the group of phyllosilicates and has the chemical formula KAl₂(AlSi₃O₁₀)(OH)₂. It is known for its characteristic thin and flaky crystals that are easy to cleave. Muscovite is found in a variety of geologic environments and plays an important role in many types of rocks, especially metamorphic and igneous rocks. Although muscovite is not known for prominent fluorescence, in rare cases it can fluoresce weakly under UV light, depending on the specific impurities and chemical composition. However, this fluorescence is usually weak and not as noticeable as other fluorescent minerals.
Brazil is known worldwide for its diverse and rich mineral deposits, including muscovite. Brazilian muscovite is often characterized by its remarkable clarity and large crystal sizes, which make it particularly valuable for both scientific studies and industrial applications.

Muscovite under daylight

Muscovite under UV light
Natrolite is a common mineral from the zeolite group, known for its needle-like crystals and striking fluorescence under UV light. Chemically, natrolite is a sodium aluminum silicate with the formula Na₂Al₂Si₃O₁₀-2H₂O. This mineral is prized for its interesting crystal structure and ability to fluoresce in different colors under UV light, with the lemon yellow fluorescence being particularly striking. The lemon-yellow fluorescence observed in natrolite from Soutěsky is particularly striking and is often caused by the presence of small amounts of activator ions such as iron or other metal ions. These ions can trigger electron transitions in the crystal lattice, leading to the emission of light in the yellow spectral range.
Soutěsky, a small town near Děčín in the northern Czech Republic, is known for its significant deposits of natrolite. The geological conditions in this region have led to the formation of natrolite crystals, which stand out due to their clear transparency and impressive fluorescence under UV light. This region is particularly famous for natrolite samples that show a strong lemon-yellow fluorescence when exposed to UV light, a characteristic that distinguishes them from natrolite from other locations.

Natrolite under daylight

Natrolite under UV light
Fluorite or fluorspar is a fascinating mineral known for its extraordinary variety of colors and its remarkable fluorescence under UV light. Chemically, it is calcium fluoride (CaF₂), a mineral from the class of simple halides that occurs in numerous geological environments. Fluorite is particularly known for its bright fluorescence in various colors, with blue being one of the most common. The fluorescence is often caused by the presence of trace elements such as europium, yttrium or lanthanum. These elements lead to electron transitions that emit light in the visible range, particularly in the blue spectrum. In some cases, fluorescence can also be due to defects in the crystal lattice that act as activation centers for luminescence.
Harrachov, a small town in the Giant Mountains in the Czech Republic, is known for its rich deposits of fluorite. The fluorite crystals found there are characterized by their clear transparency and vivid colors. The geological conditions in this region have led to the formation of fluorites, which often have beautiful crystal shapes and impressive color intensity.

Fluorite under daylight

Fluorite under UV light
Calcite is one of the most common and diverse minerals on earth and belongs to the group of carbonates with the chemical formula CaCO₃. It occurs in a variety of colors and shapes and is known for its ability to fluoresce in different colors under UV light, depending on the trace elements and impurities it contains.
Vrančice, a region in the Czech Republic, is known for its significant calcite deposits. The calcite crystals mined there are often clear or white, but can also occur in various shades of color. This region is particularly notable for the calcites, which show a strong orange fluorescence when exposed to UV light. This is often caused by the presence of manganese (Mn²⁺) as an activator. Manganese ions in the crystal lattice partially replace calcium and lead to electron transitions that emit light in the orange spectral range.

Calcite under daylight

Calcite under UV light
The fluorescence of the calcite crystal present was quite weak. The measurement was therefore carried out using a spectrometer with a focal length of 100 mm. Although this leads to a higher sensitivity, it also results in a lower spectral resolution.
The reddish fluorescence of the calcite is clearly measurable between 550 nm and 710 nm. The weak additional signal below 550 nm and above 720 nm does not originate from the calcite, but from a slight fluorescence of the LED housing itself, which was not yet corrected during the measurement.
Cerussite is a brittle, mostly transparent lead(II) carbonate mineral with the chemical formula PbCO₃ and is known for its high density, diamond-like luster and remarkable optical properties. This mineral, also known as white lead ore, belongs to the carbonate group and often forms well-formed, transparent or white crystals. Under UV light, cerussite often shows a yellow fluorescence, which is caused by the presence of trace elements or lattice defects that trigger electron transitions in the crystal lattice and emit light in the yellow region of the spectrum. In cerussite, this yellow fluorescence is often the result of activator ions such as lead itself or other foreign elements contained in the lattice.
Stříbro, a historic mining town in the Czech Republic, has long been known for its rich deposits of lead and silver minerals. The cerussite from Stříbro typically shows a strong yellow fluorescence when exposed to UV light. Contrary to expectations, the recorded spectrum shows a rather greenish fluorescence.

Cerussite under daylight

Cerussite under UV light
 Would you like to replicate the experiment—in your laboratory or teaching environment? Feel free to contact us—we will assist you with planning, setup, calibration, and selecting the right components. Eureca offers advice based on many years of expertise in optoelectronics, optics and spectroscopy—from DIY setups to OEM solutions. Feedback is expressly welcome: Please share your experiences, results, or suggestions for improvement with us.
Would you like to replicate the experiment—in your laboratory or teaching environment? Feel free to contact us—we will assist you with planning, setup, calibration, and selecting the right components. Eureca offers advice based on many years of expertise in optoelectronics, optics and spectroscopy—from DIY setups to OEM solutions. Feedback is expressly welcome: Please share your experiences, results, or suggestions for improvement with us.
Here you can easily ask a question or inquiry about our products:
Last update: 2025-30-10
