


Neon lamps consist of a small glass housing filled with a noble gas (usually neon, sometimes argon). They have two electrodes between which a voltage is applied. With sufficient voltage, the gas ionizes and produces a faint shine (glow) around the negatively charged electrode (cathode).
Glow lamps are often used as inexpensive indicators, for example in power strips.
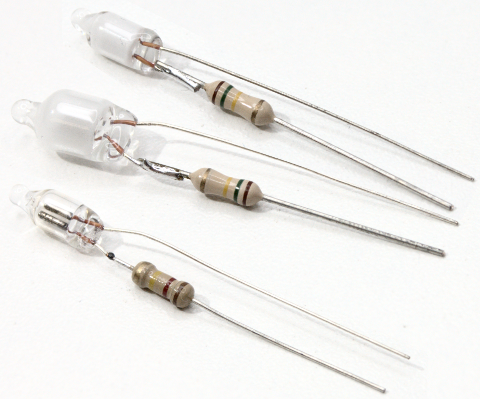
As a rule, red glow lamps only use neon to generate the emitted light. The spectrum therefore contains many typical emission lines between 585 nm and 703 nm.

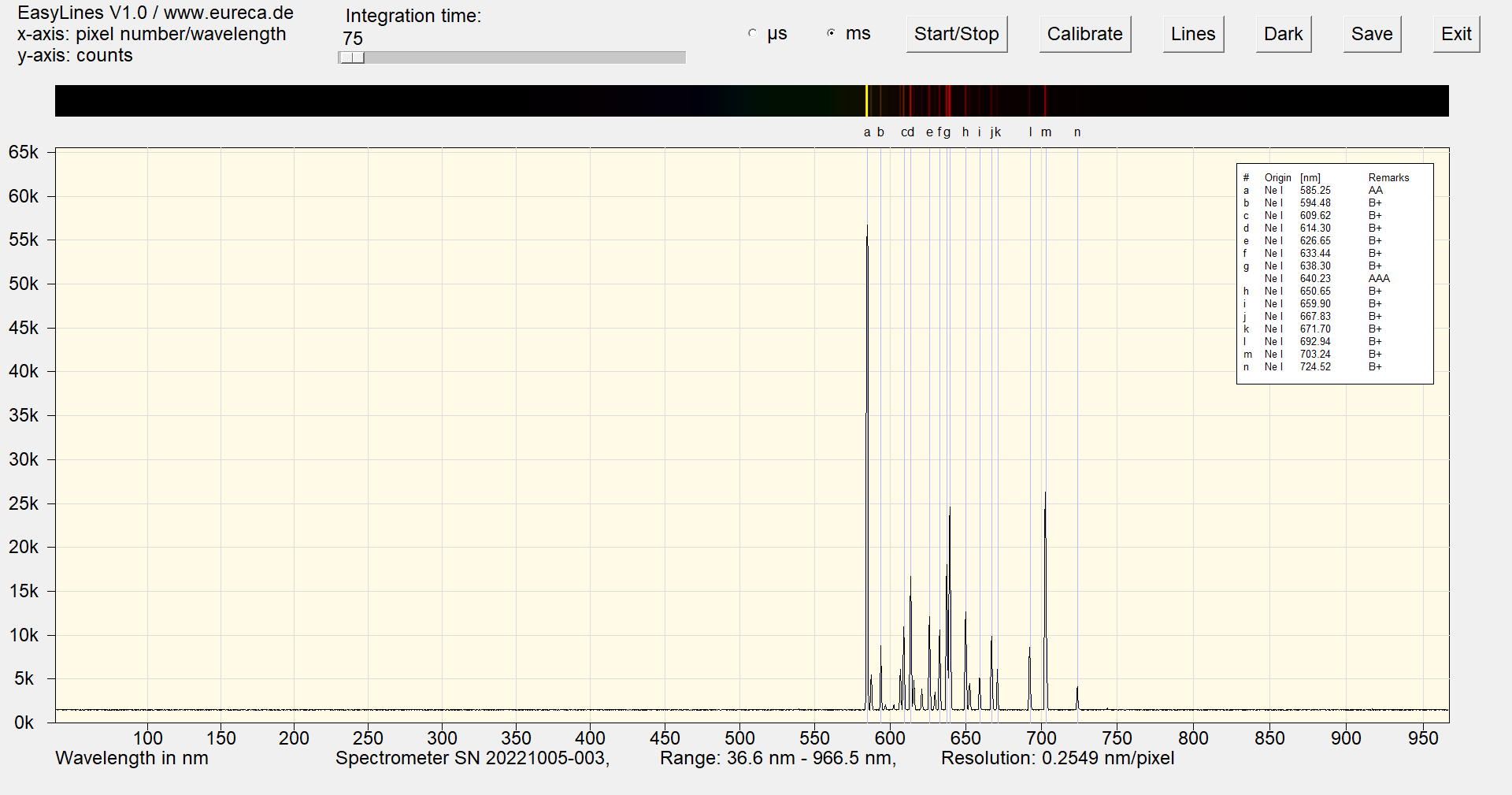
Spectrum of a red neon lamp with inserted reference values ¹
In addition to neon, this type of neon lamp also contains xenon. The latter has emission peaks in the ultraviolet, which are then converted into green light via a phosphor. However, xenon also has additional emission peaks at higher wavelengths, which can also be used to calibrate spectrometers.
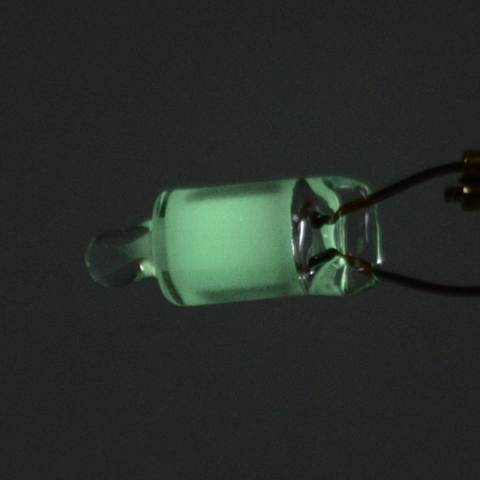
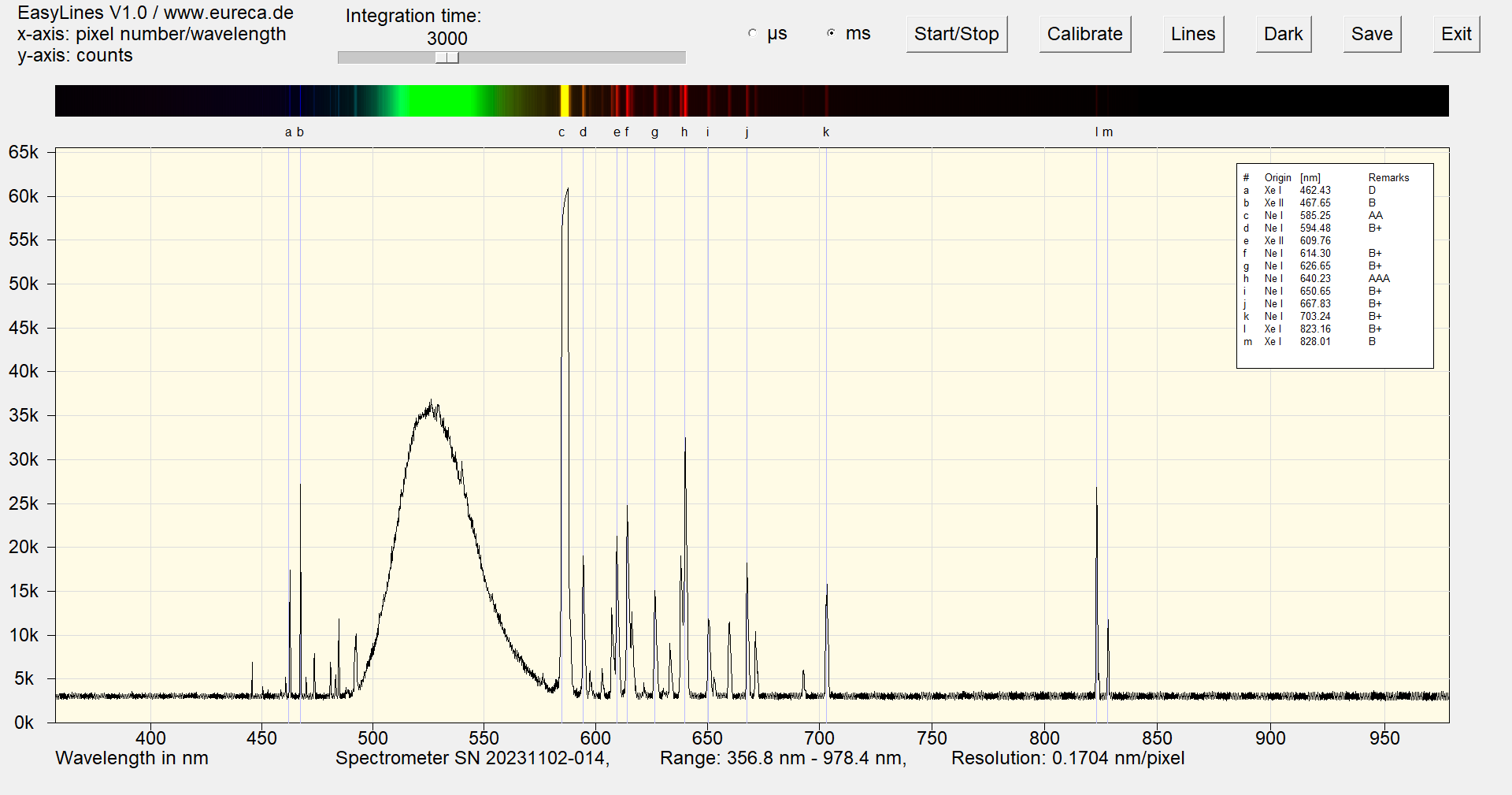
Spectrum of a green neon lamp with inserted reference values ¹
The color of this glow lamp is also produced by a corresponding phosphor. There are also emission lines of xenon and krypton in the spectrum.
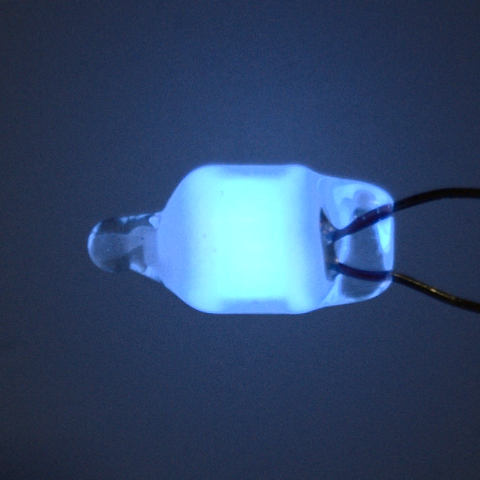
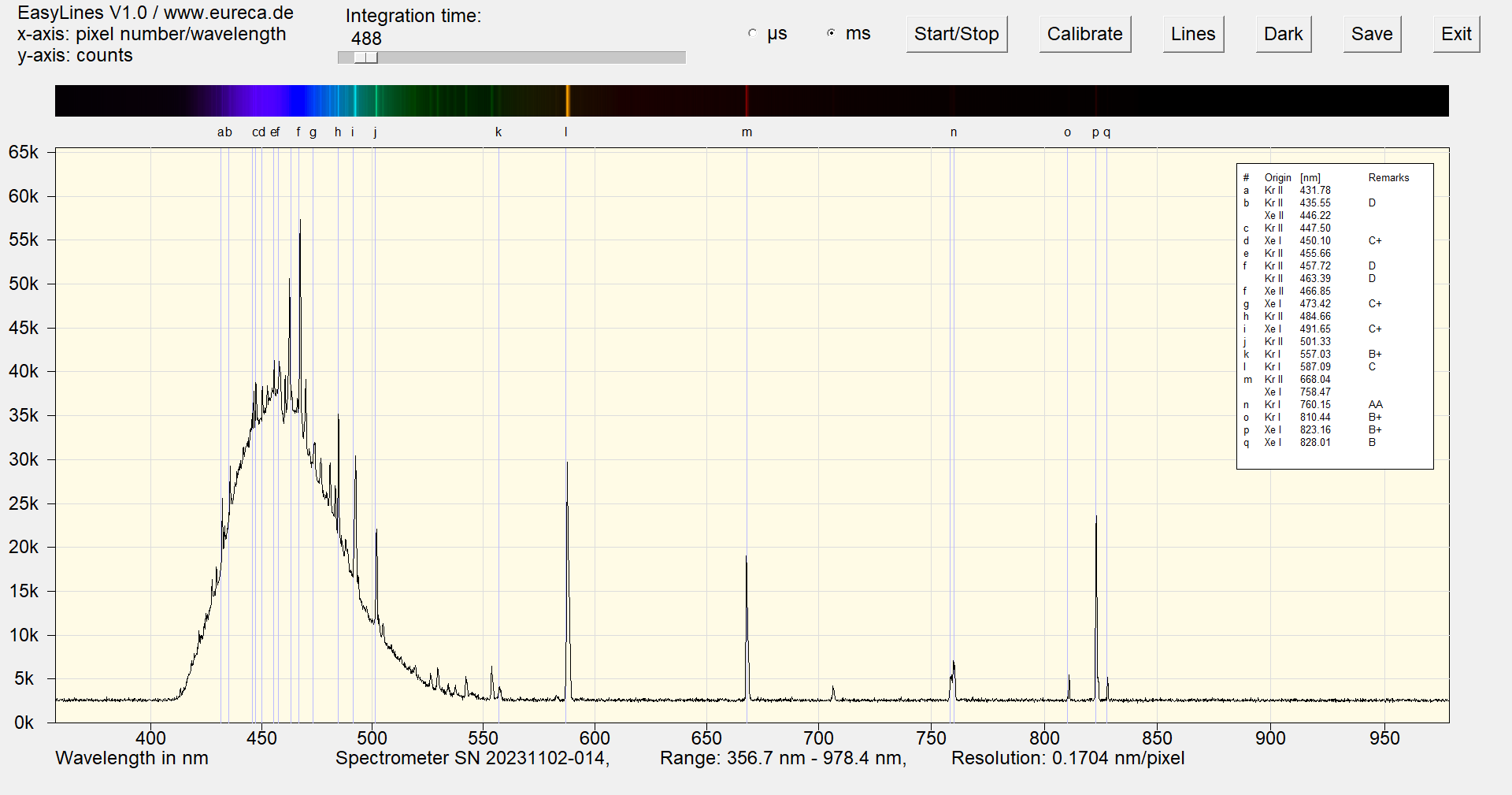
Spectrum of a blue neon lamp with inserted reference values ¹
¹ The spectra were recorded with the Czerny-Turner spectrometers from our optoelectronic consctruction kit.
The data of the superimposed emission lines are from the Atomic Spectra Database | NIST.
Kramida, A., Ralchenko, Yu., Reader, J., and NIST ASD Team (2023). NIST Atomic Spectra Database (ver. 5.11), [Online]. Available: https://physics.nist.gov/asd [2024, June 23]. National Institute of Standards and Technology, Gaithersburg, MD. DOI: https://doi.org/10.18434/T4W30F
 Would you like to replicate the experiment—in your laboratory or teaching environment? Feel free to contact us—we will assist you with planning, setup, calibration, and selecting the right components. Eureca offers advice based on many years of expertise in optoelectronics, optics and spectroscopy—from DIY setups to OEM solutions. Feedback is expressly welcome: Please share your experiences, results, or suggestions for improvement with us.
Would you like to replicate the experiment—in your laboratory or teaching environment? Feel free to contact us—we will assist you with planning, setup, calibration, and selecting the right components. Eureca offers advice based on many years of expertise in optoelectronics, optics and spectroscopy—from DIY setups to OEM solutions. Feedback is expressly welcome: Please share your experiences, results, or suggestions for improvement with us.
Here you can easily ask a question or inquiry about our products:
Last update: 2025-30-10
