


Pen-Ray light sources are compact, durable and reliable light sources in the form of a pen, which are often used in spectroscopy. They are low-pressure gas discharge lamps in a quartz tube. They are available in different fillings, including UV, visible and infrared light. This enables their use in a variety of spectroscopic applications, such as UV-Vis spectroscopy, fluorescence spectroscopy and absorption spectroscopy.

Pen-Ray light sources are small and lightweight, making them easy to handle and integrate into various experimental setups. Their compact design allows them to be used in confined laboratory environments and portable spectrometers.
Pen-Ray light sources are small and lightweight, making them easy to handle and integrate into various experimental setups. Their compact design allows them to be used in confined laboratory environments and portable spectrometers.
Furthermore, Pen-Ray light sources generate less heat compared to other light sources, especially conventional incandescent lamps. This is particularly advantageous in order to avoid thermal effects in sensitive spectroscopic measurements.
Pen-Ray light sources offer a very pure spectrum with minimal noise and low spectral contamination. This is particularly important for precise spectral analyses and the identification of specific substances.
Thanks to these advantages, Pen-Ray light sources are widely used in various spectroscopic techniques:
Pen-Ray light sources offer an excellent solution for a variety of spectroscopic applications due to their combination of compactness, stability, versatility and ease of use.
The lamps have a phenoplast end and can be operated in any position. The connection cable has a length of 300 mm.
A power supply unit is available for operation, which can both ignite and operate the lamps. Depending on the lamp type, however, the operating current must be switched between 10 mA and 18 mA using a slide switch.
The spectrum shows a whole series of emission lines in the range between 585 nm and 725 nm, which together produce the well-known characteristic reddish »neon light«.
The lines at 640.23 nm and 703.24 nm are usually particularly strong. This makes it very easy to detect neon components in luminous gases or plasmas, for example.
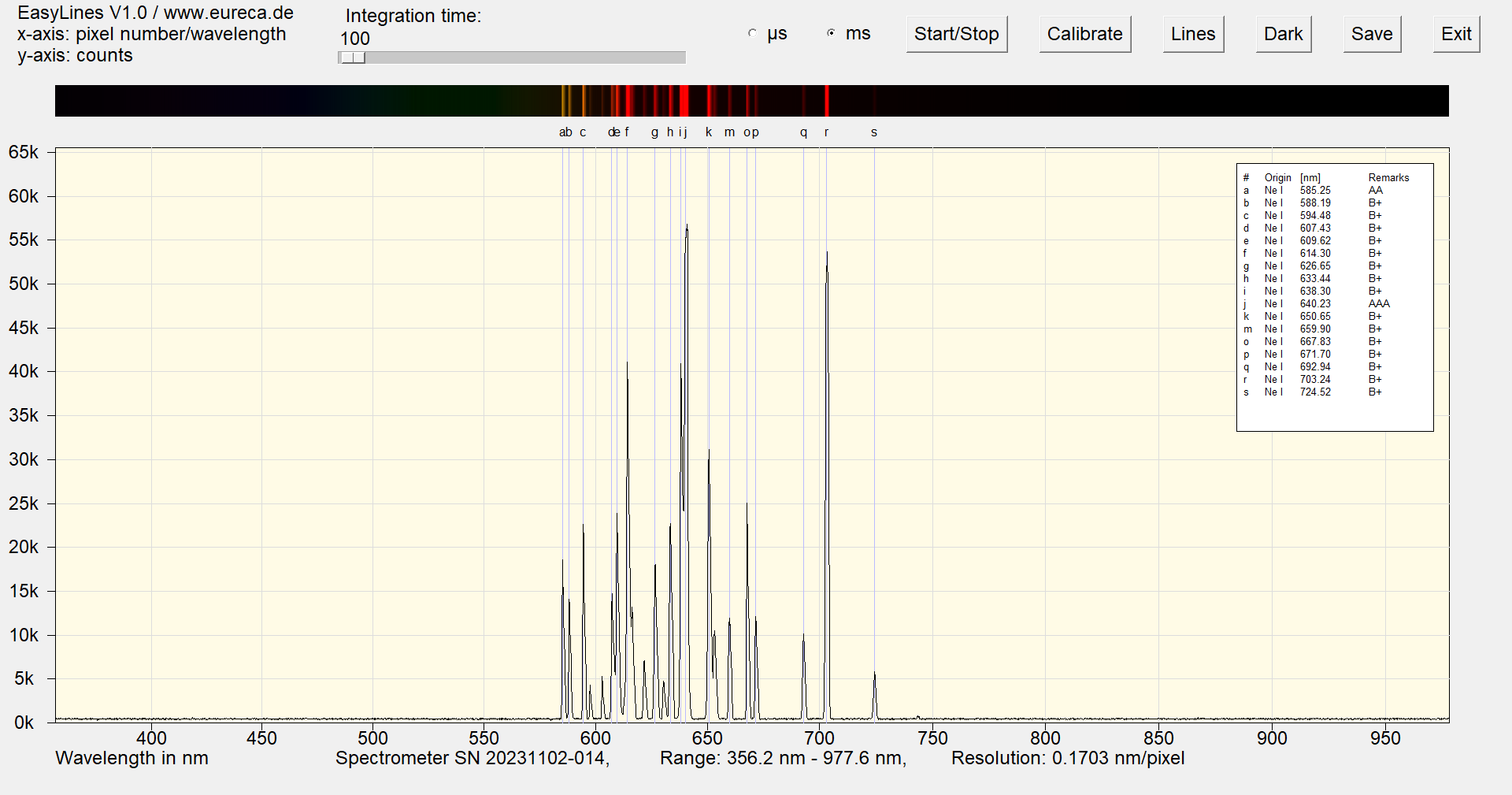
Spectrum of a neon Pen-Ray with inserted reference values ¹
The neon spectrum also contains some lines that are relatively close to each other (in relation to the resolution of the spectrometers used here) and can therefore also be used to test or adjust a spectrometer.
Would you also like to use this neon pen-ray lamp to calibrate your spectrometer? Please contact us for a quote for the following items:
The argon spectrum is dominated by a strong emission line at 763.51 nm. Together with other lines between 696 nm and 842 nm, this results in a violet hue.
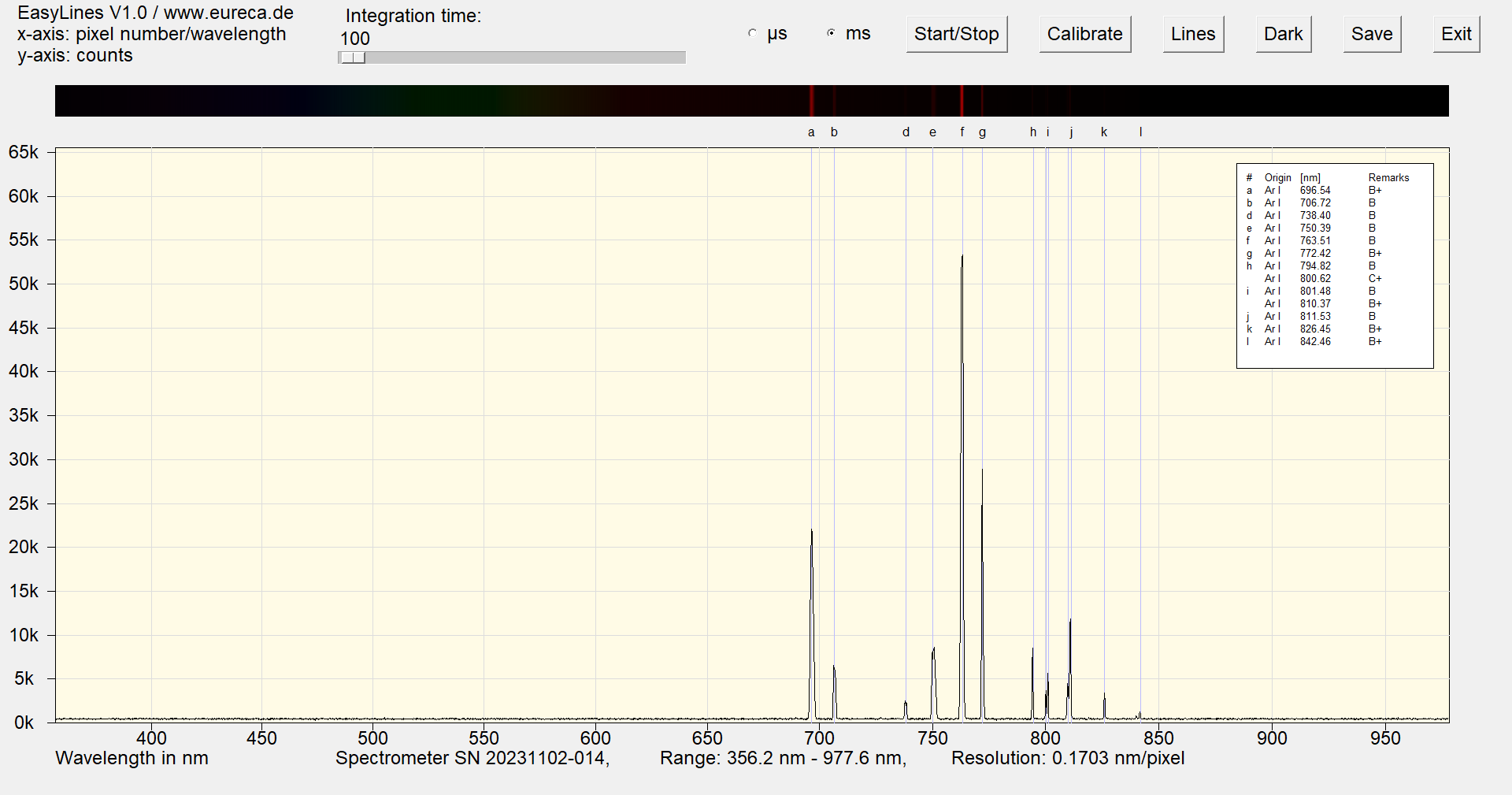
Spectrum of an argon Pen-Ray with inserted reference values ¹
Would you also like to use this argon pen-ray lamp to calibrate your spectrometer? Please contact us for a quote for the following items:
The spectrum shows the strong Kr lines in the near IR at 758.74 nm and 760.15 nm (e), which appear as a broader peak with the selected spectrometer setting and display of the plot. However, the green line at 557.03 nm (c) and the yellow line at 587.09 nm (d) are also characteristic.
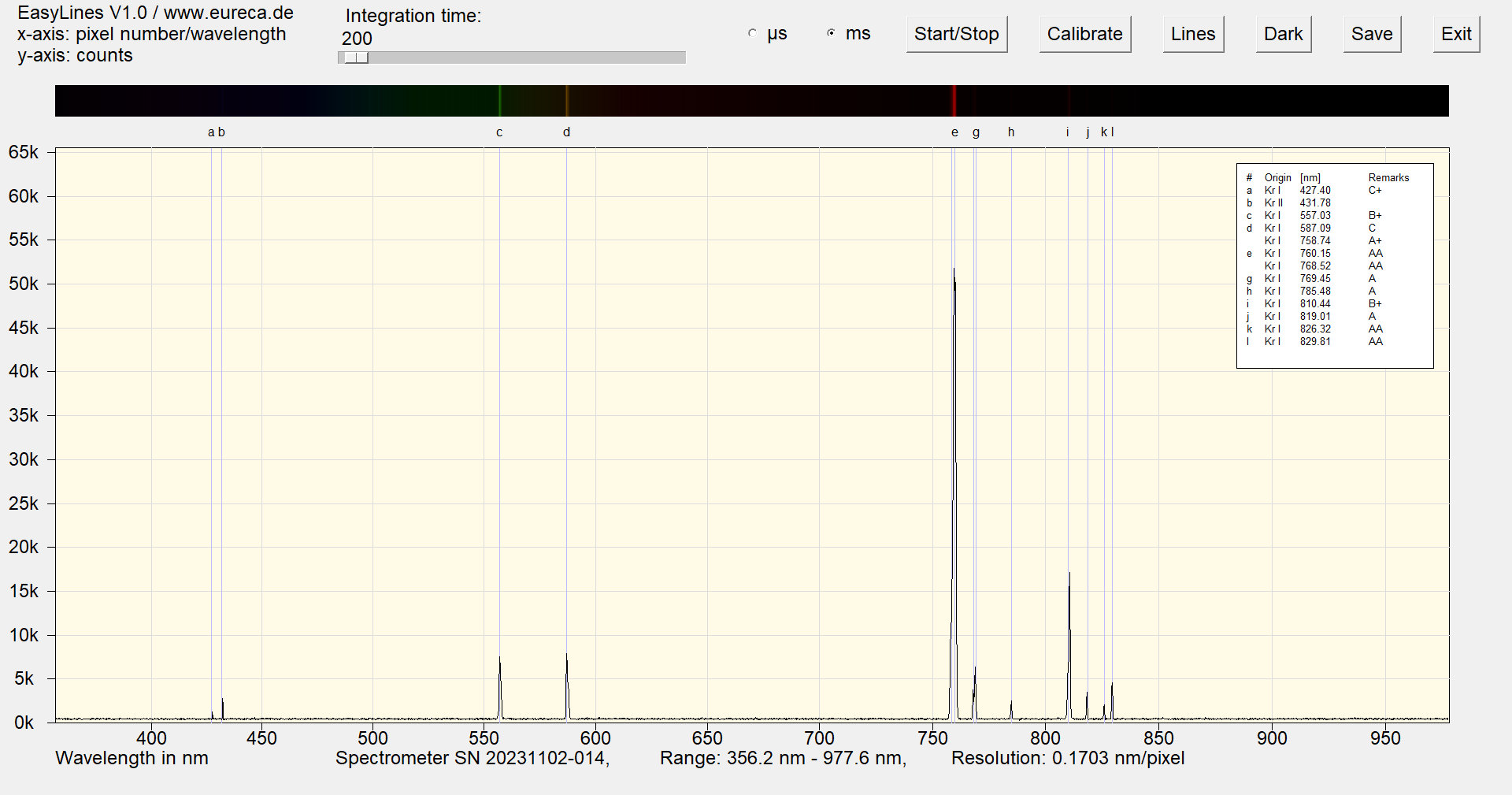
Spectrum of a krypton Pen-Ray with inserted reference values ¹
In the spectrum, the two characteristic Xe lines are found at 823.16 nm (j) and 828.01 nm (k). However, the bluish light of the xenon lamp originates from a series of lines between 450 nm and 491 nm.
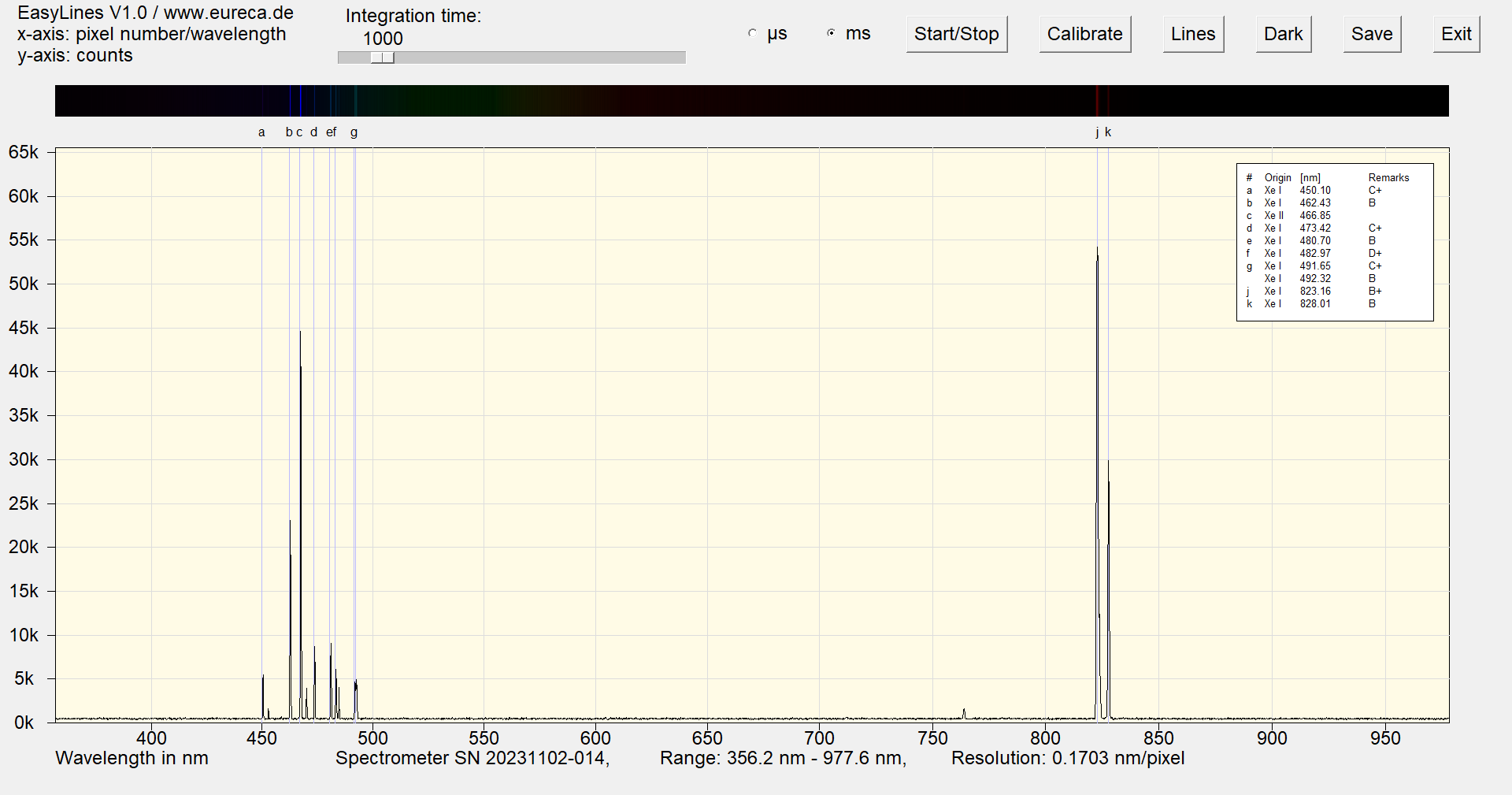
Spectrum of a xenon Pen-Ray with inserted reference values ¹
Would you also like to use this xeon pen-ray lamp to calibrate your spectrometer? Please contact us for a quote for the following items:
This lamp requires a warm-up time of 2 minutes and an additional stabilization time of 30 minutes for particularly accurate measurements. Immediately after switching on the lamp, argon lines are visible in addition to the Hg lines.
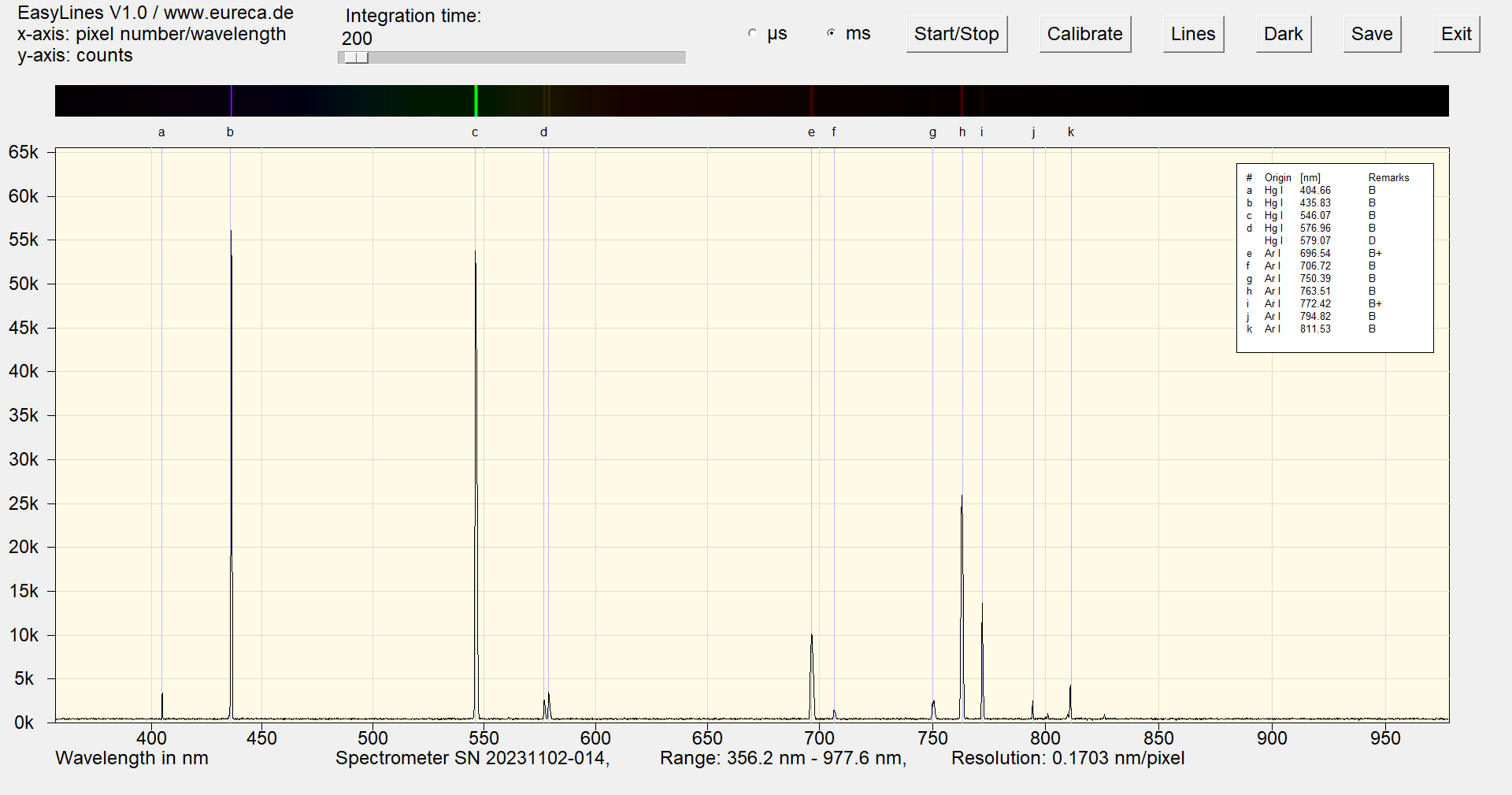
Spectrum of a mercury argon Pen-Ray with inserted reference values immediately after switching on ¹
After a few minutes of operation, the argon lines have disappeared and only the mercury lines are visible.
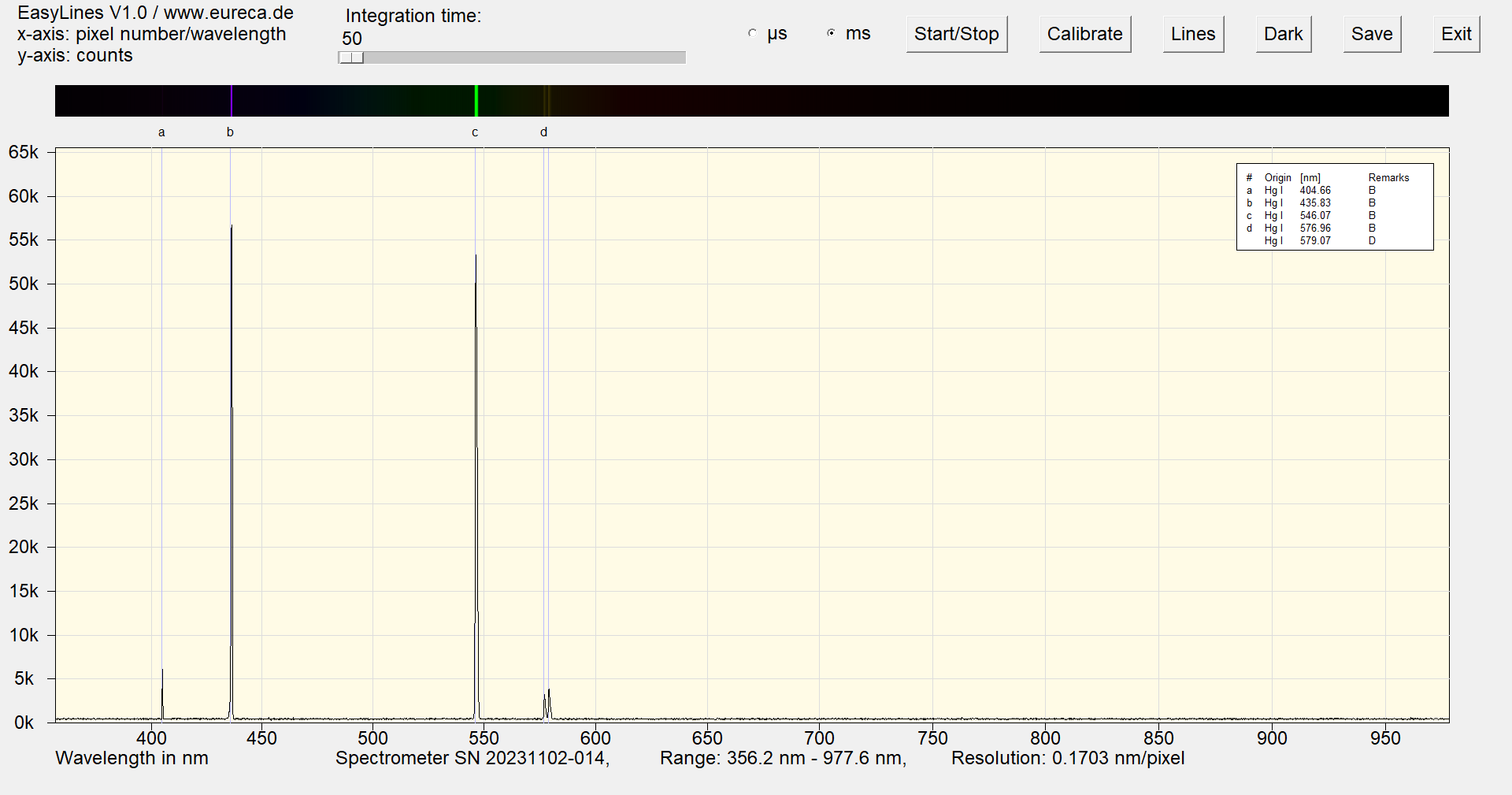
Spectrum of a mercury argon Pen-Ray with inserted reference values after a few minutes of operation ¹
Would you also like to use this mercury pen-ray lamp with argon to calibrate your spectrometer? Please contact us for a quote for the following items:
The emissions from this type of lamp react sensitively to the lamp temperature. Forced cooling, e. g. by a fan, can ensure that the neon lines remain visible even during prolonged operation.
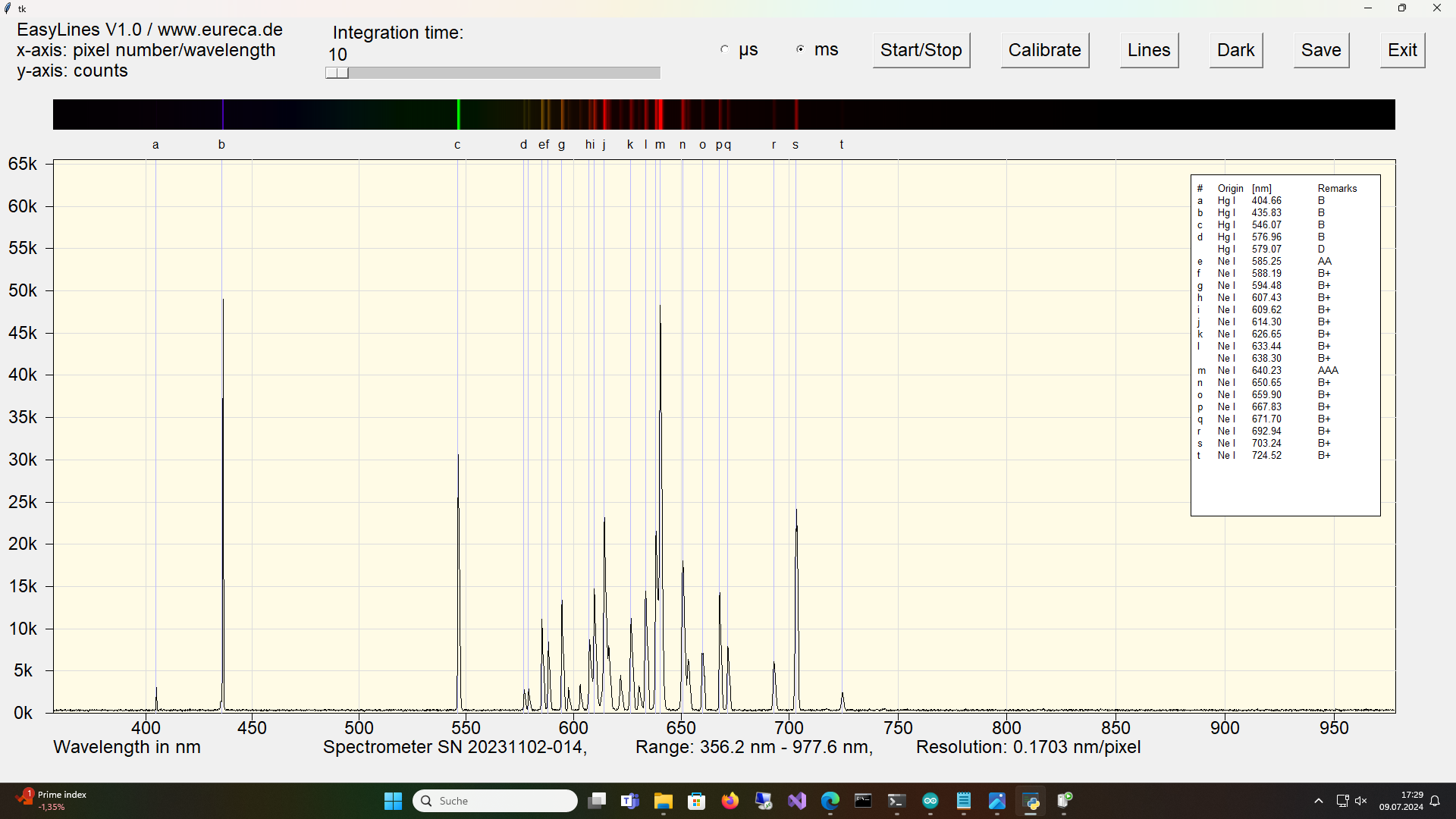
Spectrum of a mercury neon Pen-Ray with inserted reference values immediately after switching on ¹
The spectrum after a few minutes of operation corresponds to that of the pen-ray mercury (with argon) (Hg(Ar)) after a few minutes of operation: The neon lines disappear and only the characteristic mercury lines can be seen in the spectrum.
Would you also like to use this mercury pen-ray lamp with neon to calibrate your spectrometer? Please contact us for a quote for the following items:
¹ The spectra were recorded with the Czerny-Turner spectrometers from our optoelectronic consctruction kit.
The data of the superimposed emission lines are from the Atomic Spectra Database | NIST.
Kramida, A., Ralchenko, Yu., Reader, J., and NIST ASD Team (2023). NIST Atomic Spectra Database (ver. 5.11), [Online]. Available: https://physics.nist.gov/asd [2024, June 23]. National Institute of Standards and Technology, Gaithersburg, MD. DOI: https://doi.org/10.18434/T4W30F
 Would you like to replicate the experiment—in your laboratory or teaching environment? Feel free to contact us—we will assist you with planning, setup, calibration, and selecting the right components. Eureca offers advice based on many years of expertise in optoelectronics, optics and spectroscopy—from DIY setups to OEM solutions. Feedback is expressly welcome: Please share your experiences, results, or suggestions for improvement with us.
Would you like to replicate the experiment—in your laboratory or teaching environment? Feel free to contact us—we will assist you with planning, setup, calibration, and selecting the right components. Eureca offers advice based on many years of expertise in optoelectronics, optics and spectroscopy—from DIY setups to OEM solutions. Feedback is expressly welcome: Please share your experiences, results, or suggestions for improvement with us.
Here you can easily ask a question or inquiry about our products:
Last update: 2025-30-10
